V • Atomic Number 23
Vanadium
Vanadium is a silver-white transition metal with good mechanical stability and ductility. It has a very high melting point and is corrosion-resistant.
Its main application is as an additive in steel and titanium alloys to improve their strength and heat and corrosion resistance, and as a catalyst for chemicals.
The importance of vanadium redox batteries for energy storage is increasing, and with it the role of vanadium as a strategic raw material. In the EU, vanadium is on the list of critical raw materials.
China, South Africa, and Russia are the leading vanadium-producing countries.
The Canadian-Brazilian manufacturer Largo is the market leader in vanadium products, particularly high-purity vanadium pentoxide and vanadium electrolytes for batteries.
Vanadium is listed as a critical raw material in the EU and the US.
Vanadium was discovered in 1801 by Spanish-Mexican mineralogist Andrés Manuel del Río and named panchromium or erythronium. However, it was subsequently considered to be impure chromium.
The element was rediscovered in 1830 by Swedish chemist Nils Gabriel Sefström and named after Vanadis, the Scandinavian goddess of beauty and youth. The name was suggested because of the beautiful colors of vanadium compounds in solution.
English chemist Henry Enfield Roscoe first isolated the metal in 1867 by hydrogen reduction of vanadium dichloride.
American chemists John Wesley Marden and Malcolm N. Rich obtained it in 1925 in a purity of 99.7 percent by reducing vanadium pentoxide V₂O₅ with metallic calcium.
At the beginning of the 20th century, it was discovered that vanadium significantly increases the strength of steel. Henry Ford used it in the Model T (1908), which established vanadium's reputation as an alloying metal.
Today, vanadium is important for redox flow batteries, high-performance steels, and special alloys.
The most stable artificial isotopes are ⁴⁸V with a half-life of 16 days and ⁴⁹V with a half-life of 330 days. These are used as tracers. All other isotopes and nuclear isomers are very unstable and decay in minutes or seconds.
90 percent of vanadium demand comes from the steel industry, where vanadium is used in various steels and alloys for tools, axles, crankshafts, gears, and other critical components, as well as in jet engines and aircraft jets.
Vanadium is becoming increasingly important for vanadium redox flow batteries (VRFB). VRFBs are particularly suitable for large-scale renewable energy storage facilities, as they enable highly secure and environmentally friendly medium- and long-term energy storage. However, the limited availability of vanadium as a raw material is a disadvantage of VRFB technology.
Other applications include high-tech applications such as superconductors, nuclear reactors, catalysts, ceramics, and glass.
Vanadinit
China is the world leader in vanadium mining, with a 60 percent share of the global market. The most important company is Pangang in Sichuan, a major titanium ore producer.
In Russia, vanadium is produced as a by-product of steel and iron ore processing in the Urals. The EVRAZ Group is a key global player alongside VSMPO-AVISMA.
The Bushveld Complex in South Africa is another important source of vanadium mining. Brazil and Australia also mine vanadium.
Global annual production is around 100,000 tons.
Certain metals such as manganese, molybdenum, niobium (columbium), titanium, and tungsten are interchangeable to a certain extent as alloying elements in steel with vanadium.
Platinum and nickel can replace vanadium compounds as catalysts in some chemical processes.
Currently, there is no acceptable substitute for vanadium for use in titanium alloys for aerospace applications.
Physical Properties
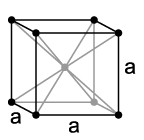
Crystal structure of vanadium, a = 302.4 pm
Vanadium is a non-magnetic, tough, malleable, and distinctly steel-blue heavy metal with a density of 6.11 g/cm³. Pure vanadium is relatively soft, but becomes harder when mixed with other elements and then has high mechanical strength. In most properties, it resembles its neighbor in the periodic table, titanium. The melting point of pure vanadium is 1910 °C, but this is significantly increased by impurities such as carbon. With a carbon content of 10%, it is around 2700 °C. Like chromium and niobium, vanadium crystallizes in a body-centered cubic crystal structure with the space group  and the lattice parameter a = 302.4 pm, as well as two formula units per unit cell.
and the lattice parameter a = 302.4 pm, as well as two formula units per unit cell.
Below a transition temperature of 5.13 K, vanadium becomes a superconductor. Like pure vanadium, alloys of vanadium with gallium, niobium, and zirconium are also superconductive. At temperatures below 5.13 K, vanadium, like the vanadium group metals niobium and tantalum, exhibits a previously unexplained spontaneous electrical polarization in tiny clusters of up to 200 atoms, which is otherwise only found in non-metallic substances.
Chemical Properties
Vanadium is a base metal and is capable of reacting with many non-metals. When exposed to air, it retains its metallic luster for weeks. When observed over longer periods of time, green rust becomes clearly visible. If vanadium is to be preserved, it must be stored under argon. When heated, it is attacked by oxygen and oxidized to vanadium(V) oxide. While carbon and nitrogen only react with vanadium when white-hot, the reaction with fluorine and chlorine takes place even at low temperatures.
Vanadium is usually stable at room temperature when exposed to acids and bases due to a thin, passivating oxide layer. It is only attacked by hydrofluoric acid and strongly oxidizing acids such as hot nitric acid, concentrated sulfuric acid, and aqua regia.
A preliminary test is provided by the phosphorus salt bead, in which vanadium appears characteristically green in the reduction flame. The oxidation flame is faintly yellow and therefore too unspecific.
Qualitative detection of vanadium is based on the formation of peroxovanadium ions. For this purpose, an acidic solution containing vanadium in the oxidation state +5 is mixed with a small amount of hydrogen peroxide. This forms the reddish-brown [V(O₂)]³⁺ cation. This reacts with larger amounts of hydrogen peroxide to form the faint yellow peroxovanadium acid H₃[VO₂(O₂)₂].
Vanadium can be determined quantitatively by titration. To do this, a sulfuric acid solution containing vanadium is oxidized to pentavalent vanadium with potassium permanganate and then back-titrated with an iron(II) sulfate solution and diphenylamine as an indicator. It is also possible to reduce the pentavalent vanadium present with iron(II) sulfate to the tetravalent oxidation state and then perform potentiometric titration with potassium permanganate solution.
In modern analytics, vanadium can be detected using several methods. These include, for example, atomic absorption spectrometry at 318.5 nm and spectrophotometry with N-benzoyl-N-phenylhydroxylamine as a color reagent at 546 nm.
Biological significance: Vanadium compounds have various biological significance. Vanadium is characterized by the fact that it occurs both anionically as vanadate and cationically as VO₂⁺, VO²⁺, or V³⁺. Vanadates are very similar to phosphates and have correspondingly similar effects. Since vanadate binds more strongly to suitable enzymes than phosphate, it is able to block and thus control phosphorylation enzymes. This affects, for example, sodium-potassium ATPase, which controls the transport of sodium and potassium in cells. This blockage can be quickly reversed with desferrioxamine B, which forms a stable complex with vanadate. Vanadium also influences glucose uptake. It is able to stimulate glycolysis in the liver and inhibit the competing process of gluconeogenesis. This leads to a reduction in blood glucose levels. Therefore, research is being conducted to determine whether vanadium compounds are suitable for the treatment of type 2 diabetes mellitus. However, no clear results have been found yet. In addition, vanadium also stimulates the oxidation of phospholipids and suppresses the synthesis of cholesterol by inhibiting squalene synthase, a microsomal enzyme system in the liver. Consequently, a deficiency causes increased concentrations of cholesterol and triglycerides in the blood plasma.
In plants, vanadium plays a role in photosynthesis. It is able to catalyze the reaction for the formation of 5-aminolevulinic acid without enzymes. This is an important precursor for the formation of chlorophyll.
Vanadium-containing enzymes occur in some organisms; for example, some species of bacteria have vanadium-containing nitrogenases for nitrogen fixation. These include species of the genus Azotobacter and the cyanobacterium Anabaenavariabilis. However, these nitrogenases are not as efficient as the more common molybdenum nitrogenases and are therefore only activated in the event of molybdenum deficiency. Other vanadium-containing enzymes are found in brown algae and lichens. These possess vanadium-containing haloperoxidases, which they use to build chlorine, bromine, or iodine organic compounds.
The function of vanadium, which is present in large quantities in sea squirts as metalloproteins called vanabins, is not yet known. It was originally assumed that vanadium serves as an oxygen transporter similar to hemoglobin; however, this has been proven to be incorrect.
Hazards
Like other metal dusts, vanadium dust is flammable. Vanadium and its inorganic compounds have been shown to be carcinogenic in animal experiments. They are therefore classified as carcinogenic category 2. If vanadium dust is inhaled by workers in metal smelting over a long period of time, it can lead to a condition known as vanadism. This recognized occupational disease can manifest itself in mucous membrane irritation, green-black discoloration of the tongue, and chronic bronchial, lung, and intestinal diseases.
Compounds: Vanadium can occur in compounds in various oxidation states. The most common states are +5, +4, +3, and +2, while +1, 0, −1, and −3 are less common. The most important and stable oxidation states are +5 and +4.
Aqueous solution
In aqueous solution, vanadium can easily be converted to different oxidation states. Since the various vanadium ions have characteristic colors, this results in color changes.
In acidic solution, pentavalent vanadium forms colorless VO₂⁺ ions, which initially become blue tetravalent VO²⁺ ions upon reduction. The trivalent state with V³⁺ ions is green in color, while the lowest state achievable in aqueous solution, the divalent V²⁺ ion, is gray-violet.
Oxygen compounds
The most important and stable vanadium-oxygen compound is vanadium(V) oxide V₂O₅. This orange-colored compound is used in large quantities as a catalyst for sulfuric acid production. It acts as an oxygen carrier and is reduced during the reaction to another vanadium oxide, vanadium(IV) oxide VO₂. Other well-known vanadium oxides are vanadium(III) oxide V₂O₃ and vanadium(II) oxide VO.
In alkaline solution, vanadium(V) oxide forms vanadates, salts with the anion VO₄³⁻ . In contrast to the analogous phosphates, however, the vanadate ion is the most stable form; hydrogen and dihydrogen vanadates as well as free vanadic acid are unstable and are only known in dilute aqueous solutions. When basic vanadate solutions are acidified, polyvanadates are formed instead of hydrogen vanadates, in which up to ten vanadate units are combined. Vanadates are found in various minerals, examples being vanadinite, descloisite, and carnotite.
Halogen compounds
Vanadium forms a variety of compounds with the halogens fluorine, chlorine, bromine, and iodine. In the oxidation state +5, only one compound is known, vanadium(V) fluoride. In the oxidation states +4, +3, and +2, compounds exist with all halogens; only with iodine are compounds known in the states +2 and +3. However, only the chlorides vanadium(IV) chloride and vanadium(III) chloride are technically relevant. Among other things, they serve as catalysts for the production of ethylene-propylene-diene rubber.
Vanadium oxide chlorides
Vanadium also forms mixed salts with oxygen and chlorine, known as vanadium oxide chlorides. Vanadium(III) oxide chloride, VOCl, is a yellow-brown, water-soluble powder. Vanadium(IV) oxide chloride, VOCl₂, which is used in photography and as a textile dye, consists of green, hygroscopic crystal plates that dissolve in water with a blue color. Vanadium(V) oxide chloride, VOCl₃, is a yellow liquid that is very easily hydrolyzed by water. VOCl₃ serves as a catalyst component in low-pressure ethylene polymerization.
Other vanadium compounds
In organic vanadium compounds, vanadium reaches its lowest oxidation states of 0, −I, and −III. Metallocenes, known as vanadocenes, are particularly important here. These are used as catalysts for the polymerization of alkynes.
Vanadium carbide VC is used in powder form for plasma spraying and plasma powder welding, among other things. Vanadium carbide is also added to hard metals to reduce grain growth. This produces so-called cermets, which are particularly hard and wear-resistant.
| General Information | ||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Name, Symbol, Atomic Number | Vanadium, V, 23 | |||||||||||||||||||||||||||||||||
| Series | Übergangsmetalle | |||||||||||||||||||||||||||||||||
| Group, Period, Block | 5, 4, d-block | |||||||||||||||||||||||||||||||||
| Appearance | Steel-gray metallic, with a bluish sheen | |||||||||||||||||||||||||||||||||
| CAS Number | 7440-62-2 | |||||||||||||||||||||||||||||||||
| Mass Fraction in Earth’s Crust | 0,041 % | |||||||||||||||||||||||||||||||||
| Atomic Properties | ||||||||||||||||||||||||||||||||||
| Atomic Mass | 50,9415 u | |||||||||||||||||||||||||||||||||
| Atomic Radius (calculated) | 135 (171) pm | |||||||||||||||||||||||||||||||||
| Covalent Radius | 153 pm | |||||||||||||||||||||||||||||||||
| Electron Configuration | [Ar] 3d3 4s2 | |||||||||||||||||||||||||||||||||
| 1. Ionization Energy | 650,9 kJ/mol | |||||||||||||||||||||||||||||||||
| 2. Ionization Energy | 1414 kJ/mol | |||||||||||||||||||||||||||||||||
| 3. Ionization Energy | 2830 kJ/mol | |||||||||||||||||||||||||||||||||
| 4. Ionization Energy | 4507 kJ/mol | |||||||||||||||||||||||||||||||||
| 5. Ionization Energy | 6298,7 kJ/mol | |||||||||||||||||||||||||||||||||
| Physical Properties | ||||||||||||||||||||||||||||||||||
| State at 20 °C | Solid | |||||||||||||||||||||||||||||||||
| Crystal Structure | Body-centered cubic (bcc) | |||||||||||||||||||||||||||||||||
| Density | 6,11 g/cm3 (at 20 °C) | |||||||||||||||||||||||||||||||||
| Mohs Hardness | 7,0 | |||||||||||||||||||||||||||||||||
| Magnetism | Paramagnetic ( = 3,8 · 10−4) = 3,8 · 10−4) |
|||||||||||||||||||||||||||||||||
| Melting Point | 2183 K (1910 °C) | |||||||||||||||||||||||||||||||||
| Boiling Point | 3680 K (3407 °C) | |||||||||||||||||||||||||||||||||
| Molar Volume | 8,32 · 10−6 m3/mol | |||||||||||||||||||||||||||||||||
| Heat of Vaporization | 453 kJ/mol | |||||||||||||||||||||||||||||||||
| Heat of Fusion | 21,5[5] kJ/mol | |||||||||||||||||||||||||||||||||
| Speed of Sound | 4560 m/s at 293,15 K | |||||||||||||||||||||||||||||||||
| Specific Heat Capacity | 489 J/(kg · K) | |||||||||||||||||||||||||||||||||
| Electrical Conductivity | 5 · 106 A/(V · m) | |||||||||||||||||||||||||||||||||
| Thermal Conductivity | 31 W/(m · K) | |||||||||||||||||||||||||||||||||
| Chemical Properties | ||||||||||||||||||||||||||||||||||
| Common Oxidation States | +5, +4 ,+3 ,+2 | |||||||||||||||||||||||||||||||||
| Electronegativity (Pauling scale) | 1,63 | |||||||||||||||||||||||||||||||||
| Isotopes | ||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
| Safety Information | ||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
| No GHS pictograms |
H- and P-Statements H: No H-statements EUH: No EUH-statements P: No P-statements Hazard labeling (powder form)
 |
 |
| Highly flammable | Irritant |
| (F) | (Xi) |
R- and S-phrasesR: 17-36/37/38 (powder)S: 7-26-33-37-43-60 (powder)
Critical and Strategic Metals


