Na • Atomic Number 11

Sodium
Sodium is a common chemical element with the symbol Na and atomic number 11. In the periodic table of elements, it is in the third period and, as an alkali metal, in the first IUPAC group or first main group. Sodium is a pure element whose only stable isotope is 23Na.
Elemental sodium was first obtained in 1807 by Humphry Davy through molten salt electrolysis from sodium hydroxide and named sodium. This name is used in English and French, with derivatives in Romance languages and some Slavic languages. The German name Natrium is derived from the Arabic نطرون, DMG naṭrūn, natron, from the Egyptian netjerj. Sodium and derivatives thereof are used in Scandinavian, Dutch, and some Slavic languages, as well as in German. In Japanese, sodium has the German-sounding name ナトリウム Natoriumu.
Under normal conditions, sodium is a soft, silvery-white and highly reactive metal. Due to its high reactivity, metallic (elemental) sodium is stored under inert conditions, usually in paraffin oil or petroleum, and in larger quantities in airtight steel drums.
Sodium is one of the ten most common elements in the Earth's crust and occurs in numerous minerals. Seawater contains a considerable amount of sodium in the form of sodium ions.
The Egyptians in ancient times coined the term netjerj (neter) for soda obtained from soda lakes. The Greeks adopted this word as Greek νίτρον (nitron), the Romans as nitrium, and the Arabs as natrun. Sodium compounds, unlike the elemental metal, have been known for a very long time and were obtained from seawater or lakes, mined from natural deposits, and traded.
The most important sodium compound, table salt (sodium chloride), was obtained from mines or by evaporating seawater or brine from salt springs in saltworks. Trade in salt was the foundation of wealth for many cities and even shaped their names (e.g., Salzgitter, Salzburg). The Germanic name for saltworks (Hall) is preserved in place names such as Hallstatt, Hallein, Halle (Saale), Bad Hall, Bad Reichenhall, Schwäbisch Hall, Schweizerhalle, and Hall in Tirol. Other naturally occurring sodium compounds, such as sodium carbonate (soda or natron) and sodium nitrate (Chile saltpeter), were also extracted and traded since antiquity.
The production of elemental sodium was first achieved in 1807 by Humphry Davy, who electrolyzed molten sodium hydroxide (caustic soda) using Voltaic piles as a power source. As he reported to the Royal Society in London on November 19, 1807, he obtained two different metals: the one contained in soda he named sodium (a term still used in French and English today), and the other metal he called potassium. In 1811, Berzelius proposed the modern name natrium.
Large quantities of sodium chloride and other sodium compounds, such as sodium carbonate, are mined and processed. However, only a small portion of this is further processed into metallic sodium. The majority is used directly or converted into other compounds.
Sodium is the most widely used alkali metal. It is employed for technical, industrial, and laboratory purposes. In school experiments and demonstration lectures, sodium can be used — often with a sodium spoon — to generate hydrogen gas when reacted with water.
A portion of the sodium is used to produce various sodium compounds, such as sodium peroxide (used as a bleaching agent) and sodium amide, a strong base. These compounds do not occur naturally and cannot be obtained directly from sodium chloride. Sodium cyanide and sodium hydride are also synthesized from sodium. Because sodium influences the solidification structure of metals, it is used as an additive in aluminum–silicon alloys (a refinement process developed by Aladár Pácz).
Catalyst
Sodium catalyzes the polymerization of 1,3-butadiene and isoprene, which made it useful in the production of synthetic rubber. The synthetic rubber known as Buna (from Butadiene and Natrium) was the first artificial rubber in the world and was produced starting in 1937 at the Buna Works in Schkopau, Germany.
Coolant
Because sodium has a high thermal conductivity (140 W/(m·K)) — far higher than that of steel (15–58 W/(m·K)) — and combines a low melting point with a wide liquid temperature range, it is used as a coolant in several applications:
-
In internal combustion engines, sodium is used to cool exhaust valves. The valve stems are hollow and partially filled with sodium, which melts during operation and oscillates between the hot and cold ends, transferring heat away from the red-hot valve head.
-
Fast breeder reactors are cooled with liquid sodium. In these reactors, the fast neutrons produced during fission must not be slowed down, as water would do; therefore, sodium — which does not act as a moderator — is used instead. The heat is then transferred through a secondary sodium loop to a steam generator, which powers a turbine.
Light Production
Sodium vapor lamps utilize the characteristic yellow light emitted by sodium vapor in an electric discharge. Due to their high luminous efficiency, they are widely used for street lighting.
Reducing Agent
Some metals, such as titanium, zirconium, tantalum, and uranium, cannot be obtained by carbon reduction, as this would form stable, inseparable carbides. Instead, sodium is used as a reducing agent, along with aluminum and magnesium. Sodium is also used in the production of potassium, which cannot be extracted via carbon reduction or by electrolysis due to potassium’s high solubility in molten potassium chloride.
In organic synthesis, sodium serves as an important reducing agent. For many years, its most significant industrial use was in producing tetraethyllead from chloroethane, an antiknock additive for gasoline. Due to environmental concerns, the use of tetraethyllead has been banned or severely restricted, leading to a decline in sodium consumption. Sodium is also used in reactions such as the Birch reduction and pinacol coupling, though these are primarily of laboratory significance.
Drying Agent
Because sodium reacts even with traces of water, freshly cut or pressed sodium wire can be used to dry organic solvents, such as diethyl ether or toluene. However, this method is not suitable for halogenated solvents (e.g., methylene chloride, chloroform) due to their violent reaction with sodium.
Sodium–potassium alloys (NaK) are liquid at room temperature and serve as heat transfer agents or dehalogenation reagents in organic synthesis. NaK is also effective for final drying of already pre-dried solvents to achieve extremely low residual water content.
Electrical Conductor
In the 1960s, experiments were conducted using sodium cables encased in polyethylene insulation. However, due to its lower electrical conductivity, a sodium-based cable would need to be approximately 75% larger in diameter than a conventional copper cable.
Detection

Here is the English translation of your text about sodium (Na), including its detection, physiology, biological roles, safety, and compounds:
Detection
The qualitative detection and quantitative determination of sodium are performed atom-spectroscopically via its intense yellow flame coloration, or more precisely, through its sodium D-doublet lines at 588.99 nm and 589.59 nm.
The purely chemical detection of sodium is very difficult. Since nearly all sodium compounds are highly water-soluble, classical precipitation reactions and gravimetric determinations are hardly possible. Exceptions include yellow sodium magnesium uranyl acetate (NaMg(UO₂)₃(CH₃COO)₉·9H₂O) and colorless sodium hexahydroxoantimonate(Na[Sb(OH)₆]), both of which are sparingly soluble. A precipitation reaction using sodium–bismuth double sulfate(3Na₂SO₄·2Bi₂(SO₄)₃·2H₂O) is also possible.
Since sodium ions in aqueous solution are colorless, color reactions are rarely used. Therefore, besides ion chromatography, spectroscopic methods are the only practical means of detection.
Physiology
Sodium is an essential element for all animal organisms. In animals, sodium—together with chlorine—is the ninth most abundant element and the third most abundant inorganic ion after calcium and potassium, making it one of the macroelements. In living organisms, sodium occurs exclusively as Na⁺ ions.
In the human body, an average adult (70 kg) contains about 100 g of sodium as Na⁺ ions, with roughly two-thirdspresent as NaCl and one-third as NaHCO₃. As sodium accounts for 90% of the extracellular electrolytes, it determines the volume of interstitial fluid through its influence on the vascular volume.
Recommended and Actual Sodium Intake
According to D-A-CH reference values, the estimated minimum sodium intake for adults is 550 mg/day.
Various organizations provide recommendations for maximum intake, such as:
-
WHO: 2 g/day
-
AHA: 1.5 g/day
Actual sodium consumption often exceeds these limits due to high salt intake (2.5 g of salt ≈ 1 g of sodium).
The German National Nutrition Survey II (NVS II) found median intakes of:
-
3.2 g/day for men
-
2.4 g/day for women
However, actual intake may be even higher since questionnaire-based data are prone to error.
The gold standard for determining sodium intake is measuring sodium excretion in 24-hour urine samples.
According to the WHO, data from the INTERSALT study showed sodium excretion levels in Germany of:
-
4.1–4.5 g/day for men
-
2.7–3.5 g/day for women
Regulation of Sodium Balance
Sodium levels are tightly regulated and closely linked to water balance.
Normal serum sodium concentration ranges between 135–145 mmol/L.
-
Hyponatremia (low sodium): causes cell swelling, potentially leading to seizures or coma.
-
Hypernatremia (high sodium): causes cell shrinkage, with similar neurological effects.
Regulation involves:
-
the renin–angiotensin–aldosterone system,
-
antidiuretic hormone (ADH or vasopressin), and
-
atrial natriuretic peptide (ANP).
The kidney is the key organ in sodium regulation.
It retains water during sodium excess to dilute sodium, and excretes sodium when necessary.
Conversely, during sodium deficiency, it conserves sodium and excretes more water.
However, renal adjustment to sodium fluctuations takes time.
Distribution in Cells
Na⁺ ions are unevenly distributed across cell membranes.
Outside the cell, Na⁺ and Cl⁻ predominate; inside, K⁺ and organic anions are dominant.
These gradients generate the membrane potential, which is vital for cell survival.
To counter ion diffusion, the sodium–potassium pump (Na⁺/K⁺-ATPase) actively restores ion gradients by pumping Na⁺ out and K⁺ in, consuming energy in the process.
Functions in Nerve Cells
Na⁺ ions play a key role in the generation and propagation of nerve impulses.
At the postsynaptic membranes of neurons and at neuromuscular junctions, neurotransmitters bind to receptors that open sodium channels, allowing Na⁺ influx.
This causes depolarization, making the cell interior less negative.
If the depolarization threshold is reached, voltage-gated sodium channels in the axon open, producing a traveling action potential.
Afterward, the sodium–potassium pump restores resting potential.
Sodium in Plants
In plants, sodium plays a minor role.
While potassium is essential for all plants and most microorganisms, sodium is required only by certain C₄ and CAM plants, but generally not by C₃ plants.
However, some plants, called halophytes, thrive in saline environments (e.g., coasts or salt flats) and benefit from sodium uptake.
Examples: sugar beet, cabbage, and many C₄ grasses.
They can transport sodium into vacuoles of leaf cells, where it acts as an osmotic ion, maintaining turgor pressure and promoting cell elongation and leaf growth.
Plants that cannot compartmentalize sodium (e.g., bean and maize) are natrophobic.
If sodium accumulates in their cytosol, it displaces potassium, leading to potassium deficiency, photosynthesis inhibition, and reduced water transport.
Since most plants contain only small amounts of sodium, herbivores must obtain additional sodium chloride from natural salt deposits.
Safety Precautions
Small amounts of sodium are stored under petroleum, while larger quantities require handling systems under inert gas.
Even with protection, sodium surfaces are often covered by a layer of sodium hydroxide and sodium oxide.
Sodium fires can be extinguished with metal fire powder (salt), potassium chloride, cast-iron shavings, or, in emergencies, dry sand or cement.
However, sand and cement can react with sodium, reducing effectiveness.
Water, foam, CO₂, or halons must never be used, as they react violently with sodium, causing explosions or intensified fires.
Compounds
In compounds, sodium always occurs in the +1 oxidation state.
All sodium compounds are strongly ionic and mostly water-soluble.
Sodium salts represent some of the most important industrial chemicals, as they are cheap to produce.
Halides
Sodium chloride (NaCl) — commonly known as table salt — is the most important and abundant sodium compound.
It serves as a primary raw material for producing sodium and other sodium compounds, and is essential for human nutrition.
It is also used for food preservation and as road salt.
NaCl crystallizes in the rock-salt (NaCl) structure, typical of many salts.
Other stable halides include sodium fluoride (NaF), sodium bromide (NaBr), and sodium iodide (NaI).
Oxygen Compounds
Five sodium oxides are known:
Na₂O, Na₂O₂, NaO₂, Na₂O₃, and NaO₃.
-
Sodium oxide (Na₂O) is present in many types of glass and forms during glassmaking from sodium carbonate.
-
Sodium peroxide (Na₂O₂) is the most important oxide, used as a bleaching agent and oxygen source in diving and submarines.
The other oxides are unstable and decompose quickly.
Sodium hydroxide (NaOH), or caustic soda, is a major industrial base used in soap and dye production and in alumina extraction from bauxite.
Sulfur Compounds
Sodium forms sulfides (Na₂S, NaHS) with hydrogen sulfide, used for heavy metal precipitation.
Sodium sulfate (Na₂SO₄) is used in detergents and the Kraft paper process.
Sodium thiosulfate (Na₂S₂O₃) serves as a photographic fixer.
Hydrides
In sodium hydride (NaH) and sodium borohydride (NaBH₄), hydrogen is in the −1 oxidation state.
NaH acts as a strong, non-nucleophilic base, while NaBH₄ is used for reductions of carbonyl compounds, such as ketones (selectively enhanced in the Luche reduction).
Both release hydrogen gas upon contact with water.
Other Compounds
Sodium carbonate (Na₂CO₃) and sodium bicarbonate (NaHCO₃) are major sodium salts of carbonic acid.
They are vital for glassmaking and baking powder production, respectively.
Sodium nitrate (NaNO₃), or Chile saltpeter, serves as a fertilizer and preservative.
Organic sodium compounds are highly reactive and unstable, unlike lithium analogs.
Stable forms, such as sodium cyclopentadienide, serve as reducing agents.
Soaps are sodium or potassium salts of fatty acids produced by saponification—boiling fats with lye.
Typical fatty acids used include lauric, myristic, palmitic, stearic, oleic, and ricinoleic acids.
n the universe, sodium ranks 14th in abundance, comparable to calcium and nickel. In the emitted light of many celestial bodies—including that of the Sun—the yellow sodium D-line can be clearly detected.
On Earth, sodium is the sixth most abundant element in the Earth’s crust, making up 2.36% of its composition. Due to its reactivity, it does not occur in its elemental form but always in compounds known as sodium salts. A major reservoir of sodium is seawater: one liter of seawater contains, on average, 11 grams of sodium ions.
Common sodium minerals include albite (also called soda feldspar, NaAlSi₃O₈) and oligoclase ((Na,Ca)Al(Si,Al)₃O₈). Besides these rock-forming minerals, which belong to the feldspar group, sodium also occurs in large salt deposits. The most significant are halite (sodium chloride, commonly known as rock salt) deposits, which formed through the evaporation of seawater. These deposits represent the most important sources for obtaining sodium and its compounds. Well-known German salt mining sites include Salzgitter, Bad Reichenhall, Stade, and Bad Friedrichshall.
In addition to common sodium chloride, several other naturally occurring sodium compounds exist. For instance, sodium nitrate, or Chile saltpeter (NaNO₃), is one of the few naturally occurring nitrate minerals. However, because it is highly soluble in water, it is only found in extremely dry regions, such as the Atacama Desert in Chile. Before the invention of the Haber–Bosch process, it was the primary raw material for many fertilizers and explosives.
Sodium carbonate (Na₂CO₃) also occurs naturally in several minerals. The best known is soda (Na₂CO₃·10H₂O), which is mined in large quantities and used primarily in glass manufacturing.
In addition, there are many other sodium minerals (see also Category: Sodium minerals). One notable example is cryolite (ice stone, Na₃[AlF₆]), which, in molten form, serves as a solvent for aluminum oxide in aluminum production. Since the only known natural cryolite deposit in Greenland has been depleted, synthetic cryolite is now produced industrially.
Sodium is mainly obtained from sodium chloride, which is usually acquired through mining or by evaporating saline solutions, such as seawater. Only a small portion of sodium chloride is processed into elemental sodium; the majority is used as table salt or for the production of other sodium compounds.
The industrial production of sodium is carried out by molten-salt electrolysis of dry sodium chloride in a device known as the Downs cell (patented in 1924 by James C. Downs). To lower the melting point, a eutectic salt mixture of 60% calcium chloride and 40% sodium chloride is used, which melts at 580 °C. Barium chloride may also be added as an additional component. A voltage of approximately seven volts is applied. During electrolysis, the production of one kilogram of sodium consumes about 10 kWh of electricity, and roughly 12 kWh for the entire production process.
Formation of Sodium at the Cathode
At the cathode, molten sodium ions are reduced to elemental sodium.
Formation of Chlorine at the Anode
At the anode, chloride ions are oxidized to form chlorine gas.
Overall Reaction
2NaCl(l)→2Na(l)+Cl2(g)2NaCl(l)→2Na(l)+Cl2(g)
The cylindrical electrolysis cell contains a central graphite anode and a cylindrical iron cathode surrounding it. Above the cell is a bell-shaped hood that collects and removes the chlorine gas produced. The sodium accumulates above the cathode and is drawn off through a cooled riser tube. Any calcium formed crystallizes and sinks back into the molten mixture.
This process replaced the Castner process, in which sodium was obtained by electrolysis of molten sodium hydroxide. Although the lower melting point of sodium hydroxide (318 °C) was advantageous, it required more electrical energy. Since the introduction of the chlor-alkali molten-salt electrolysis, the price of sodium has dropped significantly. As a result, sodium has become the cheapest lightweight metal by volume. However, its cost still depends heavily on electricity prices and the market value of the chlorine produced as a byproduct.
Physical properties
odium is a silvery-white, soft, lightweight metal. In many of its properties, it lies between lithium and potassium. For example, its melting point of 97.82 °C is between that of lithium (180.54 °C) and potassium (63.6 °C). The same relationship applies to its boiling point and specific heat capacity. With a density of 0.968 g · cm⁻³, sodium is one of the least dense elements. Among the elements that are solid at room temperature, only lithium and potassium have a lower density. With a Mohs hardness of 0.5, sodium is so soft that it can be cut with a knife.
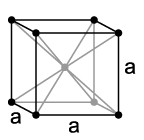
Sodium crystallizes, like the other alkali metals, in a body-centered cubic lattice with the space group Im3m (No. 229) and two formula units per unit cell. Below 51 K, it transforms into a hexagonal close-packed structure with lattice parameters a = 376 pm and c = 615 pm.
Sodium vapor consists of both individual metal atoms and dimers (Na₂). At the boiling point, about 16 % of the atoms are present as dimers. The vapor appears yellow, but when viewed through it, it shows a purple hue.
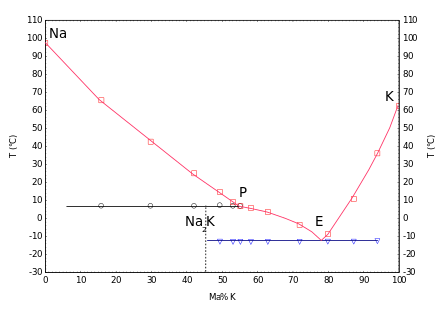
When mixed with potassium, sodium forms liquid alloys over a wide range of compositions at room temperature. The phase diagram shows an incongruently melting compound, Na₂K, which melts at 7 °C, and a eutectic point at −12.6 °C with a potassium content of 77 % (by mass).
Chemical properties
Like the other alkali metals, sodium is a very base (highly reactive) element, with a standard electrode potential of −2.71 V. It reacts easily with many other elements and, in some cases, with compounds. Its reactions with nonmetalssuch as chlorine or sulfur are particularly vigorous, producing bright yellow flames.
Oxygen, however, represents a special case. Sodium and oxygen do not react directly at room temperature or upon heating in the absence of water. In a completely moisture-free oxygen atmosphere, sodium can even be meltedwithout undergoing any reaction. However, if traces of moisture are present, sodium burns readily, forming sodium peroxide (Na₂O₂).
Reaction of Sodium with Oxygen
2Na+O2→Na2O2
The Strongly Exothermic Reaction of Sodium with Water
Sodium reacts violently and exothermically with water, producing sodium hydroxide (NaOH) and hydrogen gas (H₂). The reaction releases so much heat that the hydrogen ignites, often burning with a yellow-orange flame:
2Na+2H2O→2NaOH+H2↑
This reaction vividly demonstrates sodium’s high reactivity and the strong reducing power typical of alkali metals.

Sodium reacts with water to form sodium hydroxide (NaOH) and hydrogen gas (H₂). High-speed recordings of the reaction of alkali metals with water suggest that the process involves a Coulomb explosion—a rapid electrostatic disintegration caused by charge buildup.
Reaction of Sodium with Water
2Na+2H2O→2NaOH+H2↑
In alcohols, sodium reacts to form sodium alkoxides while releasing hydrogen gas. Because the reaction is highly exothermic, the sodium often melts during the process. When sodium is finely divided, providing a large reactive surface area, the reaction can become explosive and may ignite the released hydrogen.
Reaction of Sodium with Ethanol
2Na+2C2H5OH→2C2H5ONa+H2↑
When sodium comes into contact with chlorinated compounds such as dichloromethane, chloroform, or carbon tetrachloride, it reacts rapidly and exothermically, forming sodium chloride (NaCl) and other decomposition products.
Sodium Dissolved in Liquid Ammonia
Sodium dissolves in liquid ammonia, producing a deep blue solution. This color is caused by free electrons released from the sodium atoms into the solution. As a result, the solution conducts electricity and, in dilute form, is paramagnetic.
In a similar way, the sodium anion (Na⁻) can be isolated, for example as potassium(2.2.2-cryptand)sodium (K⁺(C222)Na⁻). This compound contains the natrid ion and acts as an extremely powerful reducing agent.
A total of 19 isotopes and 3 additional nuclear isomers of sodium are known, ranging from ¹⁸Na to ³⁷Na. Of these, only one isotope, ²³Na, occurs naturally. This makes sodium one of the 22 monoisotopic elements (elements that occur in nature with only a single stable isotope).
The most long-lived artificial isotopes are ²²Na and ²⁴Na.
-
²²Na has a half-life of 2.602 years and decays by beta-plus decay (β⁺) into ²²Ne (neon).
-
²⁴Na has a half-life of 14.957 hours and decays by beta decay into ²⁴Mg (magnesium).
Both isotopes are used as tracers in nuclear medicine. ²²Na can be produced by irradiating magnesium or aluminum targets with protons from a cyclotron for several weeks.
All other isotopes and isomers of sodium have only very short half-lives, ranging from seconds to milliseconds.
Critical and Strategic Metals

