GD • Atomic Number 64

Gadolinium
Gadolinium is a silvery-white to grayish-white shiny metal that is ductile and malleable. It belongs to the group of middle rare earth elements.
Its key property is its extremely high paramagnetic susceptibility, meaning it is strongly attracted by a magnetic field.
The most important application of gadolinium is in medicine, where it is used as a contrast agent in magnetic resonance imaging (MRI).
The element was first discovered spectroscopically in 1880 by Jean Charles Galissard de Marignac in didymium and gadolinite. In 1886, he isolated it as a white oxide from samarskite. That same year, Paul Émile Lecoq de Boisbaudran also produced gadolinium oxide and named the new element after the discoverer of the mineral gadolinite, Finnish chemist Johan Gadolin.
In nature, gadolinium only occurs in compound form. The most important industrial sources are the minerals monazite and bastnäsite.
Gadolinium is primarily found in the major rare earth ore minerals bastnäsite, monazite, and especially xenotime.
Ion-adsorption clay deposits in southern China are particularly rich in gadolinium.
After a complex separation of other gadolinium-containing elements, the oxide is converted with hydrofluoric acid into gadolinium fluoride. This is then reduced to metallic gadolinium using calcium, producing calcium fluoride as a byproduct. Remaining calcium residues and impurities are removed by an additional vacuum remelting process.
Due to its extremely strong paramagnetism, the most important application of gadolinium is as an MRI contrast agent in modern medical diagnostics.
Other properties of gadolinium make it highly relevant for niche applications.
Because of its high neutron absorption cross-section, gadolinium is used in control rods in nuclear reactors.
Gadolinium is also used in the production of phosphors for plasma displays and X-ray screens, as it activates green phosphorescence.
| General Information | |
| Name, Symbol, Atomic Number | Gadolinium, Gd, 64 |
| Series | Lanthanoid |
| Groupe, Periode, Block | La, 6, f |
| Appearance | silvery-white |
| CAS-Number | 7440-54-2 |
| Abundance in Earth's crust | 5.9ppm |
| Atomic Properties | |
| Atomic Mass | 157.25 u |
| Atomic Radius | 188 pm |
| Covalent Radius | 196 pm |
| Electron Configuration | [Xe] 4f⁷ 5d¹ 6s² |
| 1. Ionization Energy | 593.4 kJ/moll |
| 2. Ionization Energy | 1170 kJ/mol |
| 3. Ionization Energy | 1990 kJ/mol |
| 4. Ionization Energy | - |
| Physical Properties | |
| State of Matter | solid |
| Crystal Structure | Hexagonal |
| Density | 7.886 g/cm³ (at 25 °C) |
| Magnetism |
Paramagnetic (χm = 0.12) |
| Melting Point | 1585 K (1312 °C) |
| Boiling Point | 3523 K (3250 °C) |
| Molar Volume | 19.90 * 10⁻⁶ m³/mol |
| Heat of Vaporization | 305 kJ/mol |
| Heat of Fusion | 10.0 kJ/mol |
| Electrical Conductivity | 0.763 * 10⁶ A/(V·m) |
| Thermal Conductivity | 11 W/(m*K) |
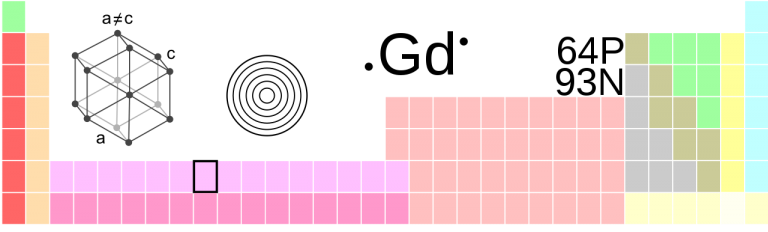
The silvery-white to grayish-white rare earth metal is ductile and malleable. Above 1508 K, its closest-packed crystal structure transforms into a body-centered cubic structure. In dry air, gadolinium is relatively stable, but in moist air it forms a non-protective, loosely adherent oxide layer that flakes off. It reacts slowly with water and dissolves in dilute acids.
Gadolinium has the highest thermal neutron capture cross-section of all known stable elements at 49,000 barns, due to its isotope Gd-157, which has a cross-section of 254,000 barns (only the unstable Xe-135 surpasses Gd-157 by about a factor of 10). However, its high burn-out rate limits its use as a control rod material in nuclear reactors.
Gadolinium is not superconducting, but ceramic high-temperature superconductors of the type Ba₂GdCu₃O₇₋ₓ with a critical temperature between 80 and 85 K are known.
Rare Earth Elements

