EU • Atomic Number 63

Europium
Europium is a silvery, lustrous heavy metal and is classified among the middle rare earth elements.
Its most important property by far is its ability to emit light in very specific, pure, and intense colors. As a result, the primary application of europium is in the production of phosphors for display and lighting technologies.
In 1890, Paul Émile Lecoq de Boisbaudran discovered unknown spectral lines in a samarium–gadolinium concentrate. The discovery of europium is credited to Eugène-Anatole Demarçay, who in 1896 suspected the presence of another element within the recently discovered samarium. In 1901, he succeeded in isolating europium. Metallic europium was not produced until several years later.
Rare Earth Elements
Europium is a rare element on Earth, with an average abundance of about 2 ppm in the continental crust.
It occurs as a minor constituent in various lanthanide-bearing minerals. Europium is found in monazite, bastnäsite, and xenotime.
In some igneous rocks, the concentration of europium is either higher or lower than expected based on the relative abundance pattern of rare earth elements normalized to chondrites. This phenomenon is known as the europium anomaly and is caused by the fact that under reducing conditions in magma, Eu³⁺ can be reduced to Eu²⁺.
After breaking down the raw materials, such as monazite or bastnäsite, using sulfuric acid or sodium hydroxide, various separation methods can be applied.
In addition to ion exchange, a process based on liquid–liquid extraction and the reduction of Eu³⁺ to Eu²⁺ is commonly used. When bastnäsite is the starting material, cerium is first removed as cerium(IV) oxide, and the remaining rare earth elements are dissolved in hydrochloric acid. A mixture of DEHPA (di(2-ethylhexyl)phosphoric acid) and kerosene is then used in liquid–liquid extraction to separate europium, gadolinium, and samarium from the other rare earth elements. These three are further separated by selectively reducing europium to Eu²⁺ and precipitating it as poorly soluble europium(II) sulfate, while the other ions remain in solution.
Metallic europium can be produced by reacting europium(III) oxide with lanthanum or mischmetal. When this reaction is carried out under vacuum, europium distills off and can thus be separated from other metals and impurities.
The first major technical application of europium was the production of europium-doped yttrium vanadate. This red phosphor, discovered in 1964 by Albert K. Levine and Frank C. Palilla, soon played a key role in the development of color television. As a result, the first rare earth mine, operating since 1954 in Mountain Pass, California, was significantly expanded to meet demand.
Europium continues to be used as a dopant in the production of phosphors for aircraft instrument displays and compact fluorescent lamps (CFLs). Phosphors containing both divalent and trivalent europium are used for different colors.
For red phosphors, europium-doped yttrium oxide (Y₂O₃:Eu³⁺) is primarily used. Previously, yttrium oxysulfide and the first significant red phosphor, yttrium vanadate:Eu³⁺, were also used. Eu²⁺ is typically used as a blue phosphor in compounds like strontium chlorophosphate (Sr₅(PO₄)₃Cl:Eu²⁺, also known as strontium chloroapatite, SCAP) and barium magnesium aluminate (BaMgAl₁₁O₁₇:Eu²⁺, BAM).
Plasma displays require phosphors that convert VUV radiation emitted by the noble gas plasma into visible light. Europium-doped phosphors are used here for both the blue and red parts of the spectrum — BAM for blue, and (Y,Gd)BO₃:Eu³⁺ for red.
Due to its ability to absorb neutrons, europium can also be used in control rods for nuclear reactors. Europium-containing control rods have been tested in various Soviet experimental reactors such as BOR-60 and BN-600.
As europium hexaboride, it is also used as a coating for oxide cathodes in thermionic emission applications.
Europium fluorescence is used as an anti-counterfeiting feature in euro banknotes.
This fluorescence property is also useful in fluorescence spectroscopy. Europium can be bound in specific complexes that selectively react and accumulate at desired locations, such as with certain proteins.
Soluble europium compounds are mildly toxic.
- Europium(III)-doped yttrium oxysulfide (Y₂O₂S:Eu³⁺) forms the red phosphor used in color CRTs (cathode ray tubes).
- Europium(II)-doped barium fluorobromide (BaFBr:Eu²⁺) is used in photostimulated luminescence (PSL) detectors.
- Eu³⁺-doped solids usually show red luminescence, while Eu²⁺ can emit across the entire optical spectrum (from UV to red), depending on the host lattice.
- Europium is used as a dopant in phosphors for light sources such as high-pressure mercury lamps and energy-saving lamps.
- Dopant in scintillation crystals (as an activator).
- Organic europium compounds are used as shift reagents in NMR spectroscopy.
- Europium-tetracycline complexes are used in fluorescence spectroscopy to detect hydrogen peroxide.
- TRFIA (time-resolved fluoroimmunoassay): Eu³⁺ ions fluoresce only briefly in water. Chelating agents are used to create a hydrophobic environment around Eu³⁺ ions, extending fluorescence duration. This makes it possible to distinguish europium fluorescence from other short-lived background signals in complex organic mixtures.
As a base metal, europium is one of the most reactive rare earth elements. When exposed to air, the silvery, shiny metal tarnishes immediately. At temperatures above 150 °C, it ignites and burns with a red flame to form the sesquioxide Eu₂O₃. In water, it reacts with the evolution of hydrogen to form the hydroxide.
Europium reacts with the halogens fluorine, chlorine, bromine, and iodine to form the respective trihalides. When reacting with hydrogen, non-stoichiometric hydride phases are formed, with hydrogen occupying the interstitial spaces of the metal’s crystal lattice.
Europium dissolves slowly in water but rapidly in acids, producing hydrogen gas and the colorless Eu³⁺ ion. The also colorless Eu²⁺ ion can be obtained via electrolytic reduction at the cathode in aqueous solution. It is the only divalent lanthanoid ion that is stable in aqueous solution. Europium also dissolves in liquid ammonia, forming a deep blue solution similar to alkali metals, in which solvated electrons are present.
The Eu³⁺ cation, like Sm³⁺, Tb³⁺, and Dy³⁺, can emit visible light when coordinated in a suitable complex and excited at specific wavelengths. While Eu³⁺ is colorless in aqueous solution, coordination with organic ligands possessing extended π-electron systems greatly enhances its luminescent properties via the antenna effect. The π-electrons of the ligand absorb incoming light (approx. 355 nm) and transfer the energy to the 5d electrons of Eu³⁺, which are then excited to the 4f orbitals. When these electrons return to their ground state, visible light (approx. 610 nm) is emitted.
With a density of 5.245 g/cm³, europium has an unusually low density — significantly lower than that of neighboring lanthanoids such as samarium or gadolinium, and even lower than lanthanum.
This also applies to its comparatively low melting point (826 °C) and boiling point (1440 °C), in contrast to the typical trend among the lanthanoids (e.g., gadolinium melts at 1312 °C and boils at 3000 °C). These anomalies contradict the lanthanoid contraction and are attributed to europium’s electron configuration. Because its 4f shell is half-filled, only two valence electrons are available for metallic bonding, resulting in weaker bonding forces and a significantly larger atomic radius — a phenomenon also observed in ytterbium.
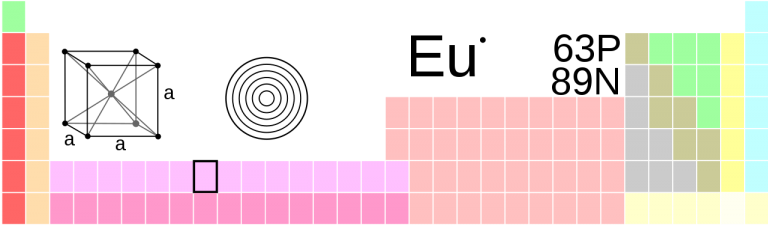
Under normal conditions, europium crystallizes in a body-centered cubic (bcc) structure with a lattice parameter of a = 455 pm. Two additional high-pressure phases are known. Unlike other lanthanoids, europium (similar to ytterbium) does not exhibit a double-hexagonal or samarium-type structure. The first phase transition occurs at 12.5 GPa, above which europium crystallizes in a hexagonal close-packed (hcp) structure with lattice parameters a = 241 pm and c = 545 pm. At pressures above 18 GPa, a third phase (Eu-III), similar to the hcp arrangement, has been identified.
Europium ions embedded in suitable host lattices show pronounced fluorescence. The emitted wavelength depends on the oxidation state. Eu³⁺ fluoresces intensely red, largely independent of the host lattice, emitting between 613 and 618 nm. In contrast, Eu²⁺ fluorescence is more sensitive to the host material. For example, emission occurs at 447 nm (blue) in barium magnesium aluminate (BaMgAl₁₁O₁₇:Eu²⁺), and at 520 nm (green) in strontium aluminate (SrAl₂O₄:Eu²⁺).
While ¹⁵³Eu is stable, evidence suggests that ¹⁵¹Eu is an alpha emitter. The lower limit of its half-life is estimated at 1.7 trillion years. Europium and its compounds are considered toxic. Metal dusts are both flammable and explosive hazards.

